Maximizing Efficiency: How CROs Can Rapidly Identify Clinicians and Centers of Excellence with Targeting Data

With over 4,200 different Clinical Research Organizations (CROs) in the U.S., CROs play a critical role in driving advancements in pharmaceutical and biotechnological fields by effectively managing and conducting clinical trials. In today’s competitive environment, the strategic use of data is paramount for CROs to swiftly pinpoint optimal clinicians and centers of excellence, ensuring expedited trial execution and enhanced outcomes.
Leveraging Data for Targeted Identification
To expedite the identification of suitable clinicians and centers of excellence, CROs can employ sophisticated data analytics with the use of tools built to analyze targeting data, such as Alpha Sophia. This approach enables them to precisely identify healthcare providers who have a proven track record in treating patients with specific diagnoses or undergoing targeted procedures. By analyzing detailed demographic and clinical data, CROs can identify specialists and facilities that align closely with the trial’s requirements. This targeted strategy not only accelerates participant recruitment but also ensures the inclusion of diverse patient populations, essential for robust clinical trial outcomes.
Enhancing Clinical Trial Recruitment and Site Selection
Effective recruitment is foundational to the success of clinical trials managed by CROs. Targeting data plays a pivotal role in identifying and engaging suitable patient populations based on detailed diagnosis and procedure counts at the hospital level. It can also provide insights into the geographical distributions and concentrations of specific diagnoses and procedures. This targeted approach not only accelerates participant recruitment but also enhances trial diversity and ensures compliance with regulatory requirements.
Moreover, strategic site selection is critical for optimizing trial outcomes. Targeting data allows CROs to identify regions with concentrated patient pools and robust medical infrastructure. By identifying centers of excellence for their targeted procedures or diagnoses, CROs can minimize logistical challenges and improve overall trial efficiency.
Identifying Key Opinion Leaders
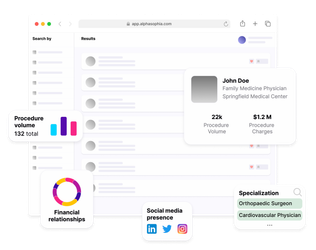
The involvement of Key Opinion Leaders (KOLs) is pivotal in establishing credibility and fostering success in clinical trials. CROs leverage data analytics to identify influential experts within specific therapeutic areas or medical disciplines. Targeting tools such as Alpha Sophia can aggregate these data points in minutes, rather than hours. This lets CROs scrutinize factors such as publication records, professional affiliations, and previous contributions to successful trials, so they can pinpoint KOLs who possess the expertise and influence needed to drive positive trial outcomes faster. Engaging these influential figures early in the trial planning process not only enhances scientific validity but also garners support from stakeholders, including regulatory bodies and potential trial participants.
Identify KOLs with Alpha Sophia
Conclusion
In conclusion, the integration of data-driven strategies is indispensable for CROs aiming to swiftly identify clinicians and centers of excellence for clinical trials. By harnessing advanced analytics and comprehensive datasets, CROs can streamline the recruitment process, mitigate operational risks, and maintain high standards of research integrity. This proactive approach not only accelerates trial timelines but also strengthens the organization’s competitive edge in the dynamic landscape of pharmaceutical research. As technology continues to evolve, embracing data-driven decision-making will be crucial for CROs to navigate complexities and drive innovation in clinical trial management effectively.